Solutions for Life Sciences & Healthcare
From virtual simulation and regulatory data management to flexible industrial design capabilities, SOLIDWORKS enables you to design regulatory-compliant medical breakthroughs.
- Home
- Solutions for Life Sciences & Healthcare
Meeting Increasing Medical Device Demands
To manage product development risk, many medical device manufacturers are moving away from point solutions for mechanical and electrical design, simulation, Product Data Management (PDM), and technical communication, toward integrated product development platforms that address all these functions.
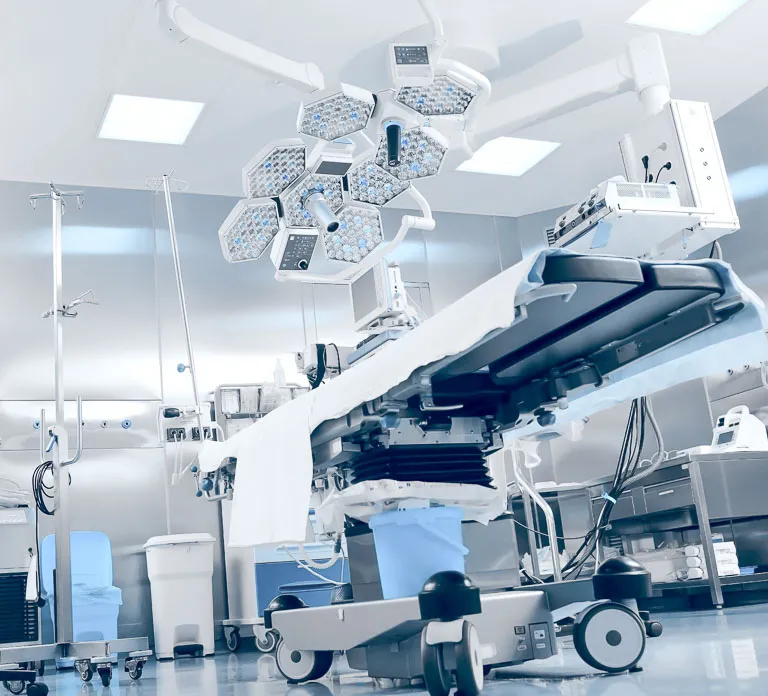
Product Definition and Concept Design
SOLIDWORKS provides easy-to-use surface modeling capabilities allowing you to create high-quality ergonomic surfaces that give your products a cutting-edge design while ensuring it is robust, fit-for-purpose, and can be manufactured.
Products
Designing with an Integrated Platform
Leverage the intuitiveness and unambiguity of 3D models over 2D drawings to define key requirements. 3D annotations can help automate downstream production procedures and are useful for organic shapes as they define 3D features not 2D geometries.
Products
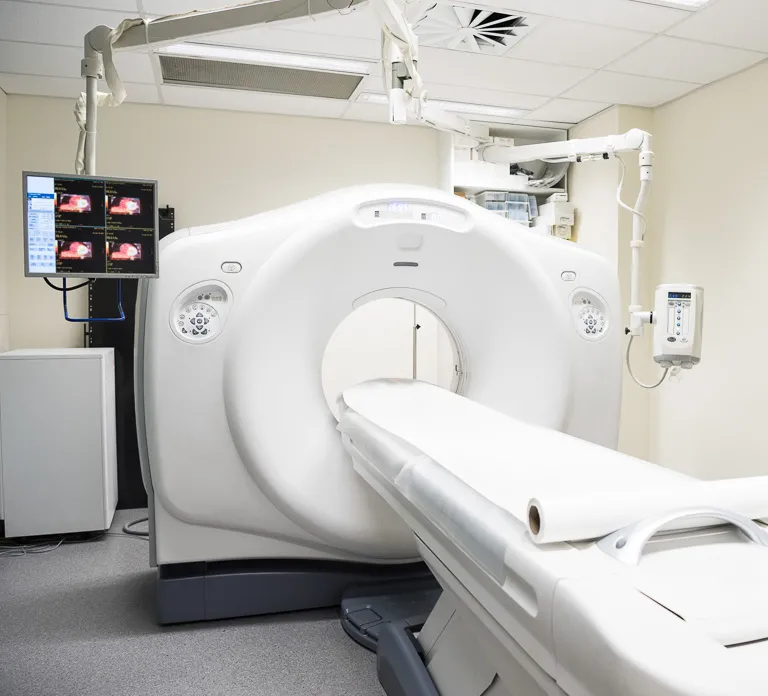

Prototyping, Testing, and Manufacturing
Prototyping and testing prior to trials is an FDA regulatory requirement. Medical product developers need access to the latest prototyping technologies. The SOLIDWORKS Manufacturing Network provides access to the best rapid prototyping technologies.
Products
Documenting Regulatory Compliance
Reduce the time and expense required to manage, archive, and track design documentation and cost-effectively automate documentation to securely archive and organize multiple versions of projects.
Products
Ready to get started with SOLIDWORKS solutions for Life Sciences & Healthcare ?
Contact us today and experience the SOPAN Infotech advantage.

